ScotSTAR Paediatric Retrieval Service tested and approved SleepAngel® Medical NICU mattresses as safety upgrade for new-born infant transport both in term of tissue viability and infection prevention.
SleepAngel Medical bedding range includes unique barrier components and PneumaPure® filter technology – invented and used to fight healthcare acquired infections and improve safety for patients and caregivers.
 ScotSTAR Paediatric Retrieval Service is a dedicated paediatric intensive care transport service based at Glasgow Airport. As the neonatal transport is an extension of the NICU, the same care philosophy needs to be delivered (1).
ScotSTAR Paediatric Retrieval Service is a dedicated paediatric intensive care transport service based at Glasgow Airport. As the neonatal transport is an extension of the NICU, the same care philosophy needs to be delivered (1).
ScotSTAR connected with SleepAngel Medical to find the best solution for new-born transport with focus of infection transmission and tissue viability, as infections and antimicrobial resistance pose a profound threat to the hospital’s most vulnerable inpatients.
Ann Marie Wilson, Former Head of Service, Neonatal Transport, ScotSTAR: “I had been looking for a new mattress for the neonatal transport incubator for some time, and a chance conversation with an adult retrieval colleague made me aware of SleepAngel and their products. I met up with Steven Lardner to explain what we need to be able to support our patient group during transport and the challenges we faced with moving this vulnerable population in road and air ambulances. As a result, 3 prototype mattresses were developed to test in road and air transport and the babies’ physical parameters were observed – If they settled during transfer, changes in temperature and similar. “
Infection prevention and tissue viability is especially important as many NICU new-borns are premature and, as a result, their immune systems are immature and weak. Those babies are also typically in the hospital for prolonged periods of time. Bacterial infections are the most common NICU infections and they are mainly either infections that are acquired during the labour and birth process or hospital-acquired infections that babies contract while they are patients in the NICU.
ScotSTAR evaluation
During the evaluation the aim was to find the best solution to bring together already proven PneumaPure® filter technology and barrier functionalities with filling materials that would consider the frail skin tissue of infants.
ScotSTAR evaluation marked SleepAngel Medical neonatal pads as safe, easy to clean and suitable for small patients and the technical conditions
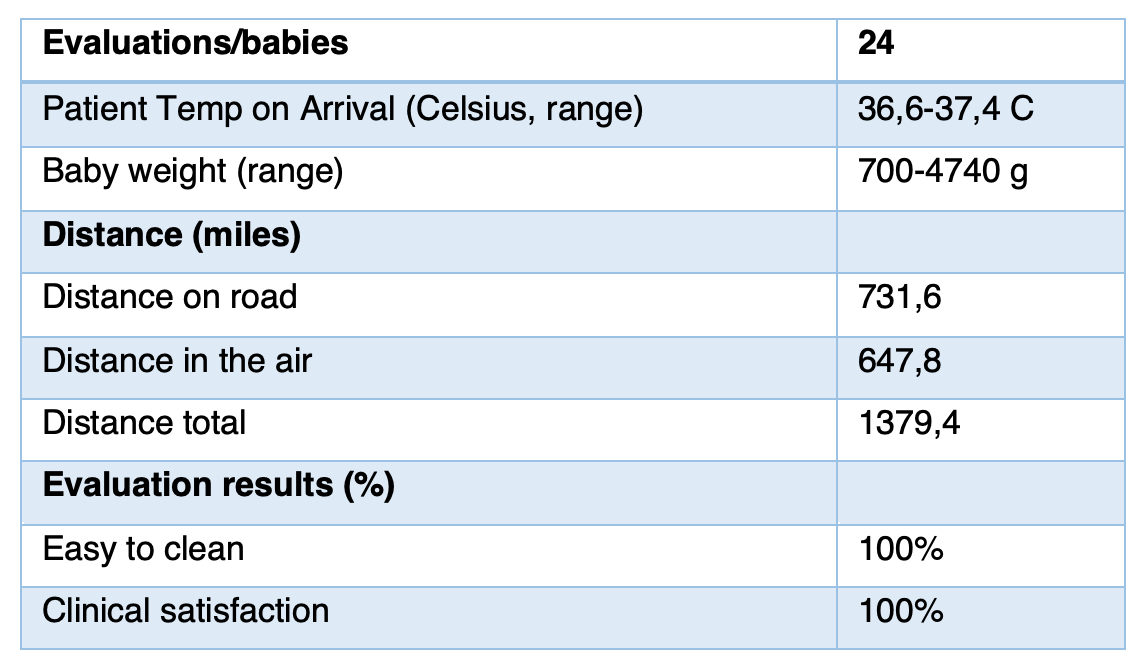
Evaluation sample size represented the user market and was adequate:
- • Sample included babies from smallest (700g) through to largest (4kg) babies
- • SleepAngel mattress was able to conduct heat from the incubator and maintain a safe and comfortable temperature environment for the baby
- • Neonatal pad was tested on road in ambulance and in aircraft
Ann Marie Wilson, ScotSTAR: “All the staff reported that the babies remained settled, and that the mattress was easy to use and most importantly clean with no deterioration in the mattress surface caused with the incubator heating, as this had been an issue with the previous ones available. The PneumaPure filter system used also is a huge infection control bonus.”
Ann Marie Wilson, ScotSTAR: “Working with SleepAngel to develop these mattresses ensured that the comfort, safety and care of our patients has been improved and this can only benefit patient care in the future.”
New NICU product range
 Creating a product range for neonatal department includes many challenges according to Lyane Lind, SleepAngel Medical R&D Director:
Creating a product range for neonatal department includes many challenges according to Lyane Lind, SleepAngel Medical R&D Director:
“The application of PneumaPure® filter technology to neonatal care has given SleepAngel its proudest and humblest moment. SleepAngel neonatal barrier bedding – specifically neonatal transport incubator mattress – is clinically proven barrier to pathogens, allergens and mould and provides protection from the risks posed by contaminated bedding (mattresses, blankets, toppers, pillows etc.). With the neonatal transport incubator mattress, we have put special focus to tissue viability and considering the very fragile patients in focus.”
The problem behind the innovation
The hospital bed is comprised of different components, which pose a potential risk of infection for the patient and care givers if not adequately decontaminated. Design of bedding products (such as pillows, mattresses, positioners, blankets, top mattresses) have not provided an adequate barrier to internal product contamination. Thread, needle perforations, air vents, zippers all allow air and liquid borne pathogen ingress. Contaminated bedding then acts as a reservoir and potential vector for infection.
The Solution: SleepAngel Barrier bedding with PneumaPure® filter technology
PneumaPure® filter is a highly specialised nano-porous filter composite (i.e., composed of extremely small pores) that functions as an effective barrier to pathogens including bacteria, virus, and fungus as well as to the ingress of liquid, while remaining highly air-permeable. Using this advanced technology in the design and manufacture of pillows, mattresses and other cushioning devices, results in a hermetically sealed cushioning device that is a clinically proven barrier to pathogen ingress yet is well ventilated, breathable, and comfortable.
SleepAngel Medical is a patented bedding range, clinically proven to block pathogens*, allergens, microbes, and mould. It is the world’s first barrier bedding range to combine absolute barrier protection with airflow – for user comfort.
Technology is patent protected globally, independently tested by Airmid Healthgroup. Product range includes pillows, mattresses, positioners, blankets, top mattresses etc. SlepAngel® Medical is produced by Gabriel Scientific in Estonia. All soft surface products include unique PneumaPure filter technology.
* Tested pathogens include MRSA, C. Difficile, E. coli, Pseudomonas aeruginosa, Candida albicans, Aspergillus niger, Norovirus, Influenza Type A, Adenovirus 5, Coronavirus 229E (HcoV 229E)
Additional information
- ScotSTAR: https://www.snprs.scot.nhs.uk
- SleepAngel Medical. SleepAngel-Medical.com
- Neonatal pads, images: link
Sources
- https://www.sciencedirect.com/topics/medicine-and-dentistry/neonatal-transport
- https://mft.nhs.uk/app/uploads/sites/4/2018/04/Welcome-to-NICU-March-2017.compressed.pdf
- https://www.yalemedicine.org/conditions/preventing-infections-in-the-nicu
ScotSTAR images via https://www.snprs.scot.nhs.uk
Download the news here: SleepAngel NICU mattresses approved by ScotSTAR retrieval service

